-
Call Us
0086-592-7161550 -
Email us
ping@aotbattery.com -
Skype
ping@aotbattery.com
Call Us
0086-592-7161550Email us
ping@aotbattery.comSkype
ping@aotbattery.comIn the ever-evolving landscape of energy storage technologies, solid-state batteries have emerged as a revolutionary development, attracting significant attention from researchers, industries, and consumers alike. As the world increasingly shifts towards sustainable energy solutions and the electrification of various sectors, such as transportation and consumer electronics, the demand for high-performance, safe, and efficient batteries has never been greater. Solid-state batteries hold the promise of addressing many of the limitations of traditional lithium-ion batteries, offering a plethora of advantages that could potentially reshape the future of the battery-powered world. This article will explore in detail what solid-state batteries are and delve into their numerous advantages.
I. Defining Solid-State Batteries
A solid-state battery is a type of battery that uses solid-state materials for all its key components, namely the electrodes and the electrolyte, instead of the liquid or gel-based electrolytes commonly found in traditional lithium-ion batteries. In a typical lithium-ion battery, the electrolyte is a liquid solution that contains lithium salts, which allows lithium ions to move freely between the positive and negative electrodes during the charging and discharging processes. However, in solid-state batteries, the electrolyte is a solid material, such as a ceramic, a solid polymer, or a glass-ceramic composite.
The solid-state electrolyte serves multiple crucial functions. Firstly, it acts as an ionic conductor, enabling the movement of lithium ions between the anode and the cathode, just like the liquid electrolyte in conventional batteries. Secondly, it functions as a physical separator, preventing direct electrical contact between the two electrodes, which is essential to avoid short circuits. Additionally, the solid-state electrolyte can also contribute to the overall mechanical stability and structural integrity of the battery.
There are different types of solid-state electrolytes, each with its own unique properties and characteristics. Ceramic electrolytes, such as (e.g., Li6.75La3Zr1.5Ta0.5O12, commonly referred to as LLZTO ) and lithium phosphates (e.g., Li10GeP2S12, known asLGPS), offer high ionic conductivity, which is beneficial for fast-charging and high-power applications. They also have excellent chemical and thermal stability, making them suitable for use in a wide range of operating conditions. However, ceramic electrolytes are often brittle and can be difficult to manufacture into thin, uniform films, which may pose
challenges in the battery fabrication process.
Solid polymer electrolytes, on the other hand, are more flexible and easier to process. They are typically composed of a polymer matrix, such as polyethylene oxide (PEO), doped with lithium salts. Solid polymer electrolytes have the advantage of being able to form intimate contact with the electrodes, which can enhance the electrochemical performance of the battery. However, their ionic conductivity is generally lower than that of ceramic electrolytes, especially at room temperature, which may limit their use in high-power applications. Glass-ceramic composites combine the advantages of both ceramic and polymer electrolytes to some extent, offering a balance between ionic conductivity, mechanical flexibility, and processability.
II. Advantages of Solid-State Batteries
A. Enhanced Safety
One of the most significant advantages of solid-state batteries is their superior safety profile compared to traditional lithium-ion batteries. Liquid electrolytes in lithium-ion batteries are flammable and can pose a fire and explosion risk under certain conditions. For example, if a lithium-ion battery is damaged due to physical impact, overcharging, or overheating, the liquid electrolyte can leak out, and the exposed electrodes can react with the electrolyte and the surrounding air, potentially leading to thermal runaway. Thermal runaway is a self-accelerating process where the heat generated by the battery causes further degradation of the battery components, releasing more heat and ultimately resulting in a fire or explosion.
In contrast, solid-state batteries eliminate the risk of electrolyte leakage since the electrolyte is in a solid state. The solid-state electrolyte is non-flammable, which greatly reduces the likelihood of fire and explosion. Moreover, solid-state electrolytes generally have higher thermal stability than liquid electrolytes. They can withstand higher temperatures without decomposing or reacting with the electrodes, which helps to prevent thermal runaway. For instance, ceramic solid-state electrolytes can maintain their stability even at temperatures well above 200°C, providing an additional layer of safety for the battery.
Another safety-related advantage of solid-state batteries is their ability to use lithium metal anodes. In traditional lithium-ion batteries, graphite is commonly used as the anode material. However, lithium metal has a much higher theoretical specific capacity (3860 mAh/g) compared to graphite (372 mAh/g), which means it has the potential to significantly increase the energy density of the battery. But lithium metal anodes are highly reactive and can form dendrites during the charging process in liquid-electrolyte-based batteries. These dendrites can grow through the separator and cause a short circuit between the anode and the cathode, leading to safety hazards. The solid-state electrolyte in solid-state batteries can effectively suppress the growth of lithium dendrites due to its high mechanical strength, allowing for the safe use of lithium metal anodes and unlocking the potential for higher-energy-density batteries.
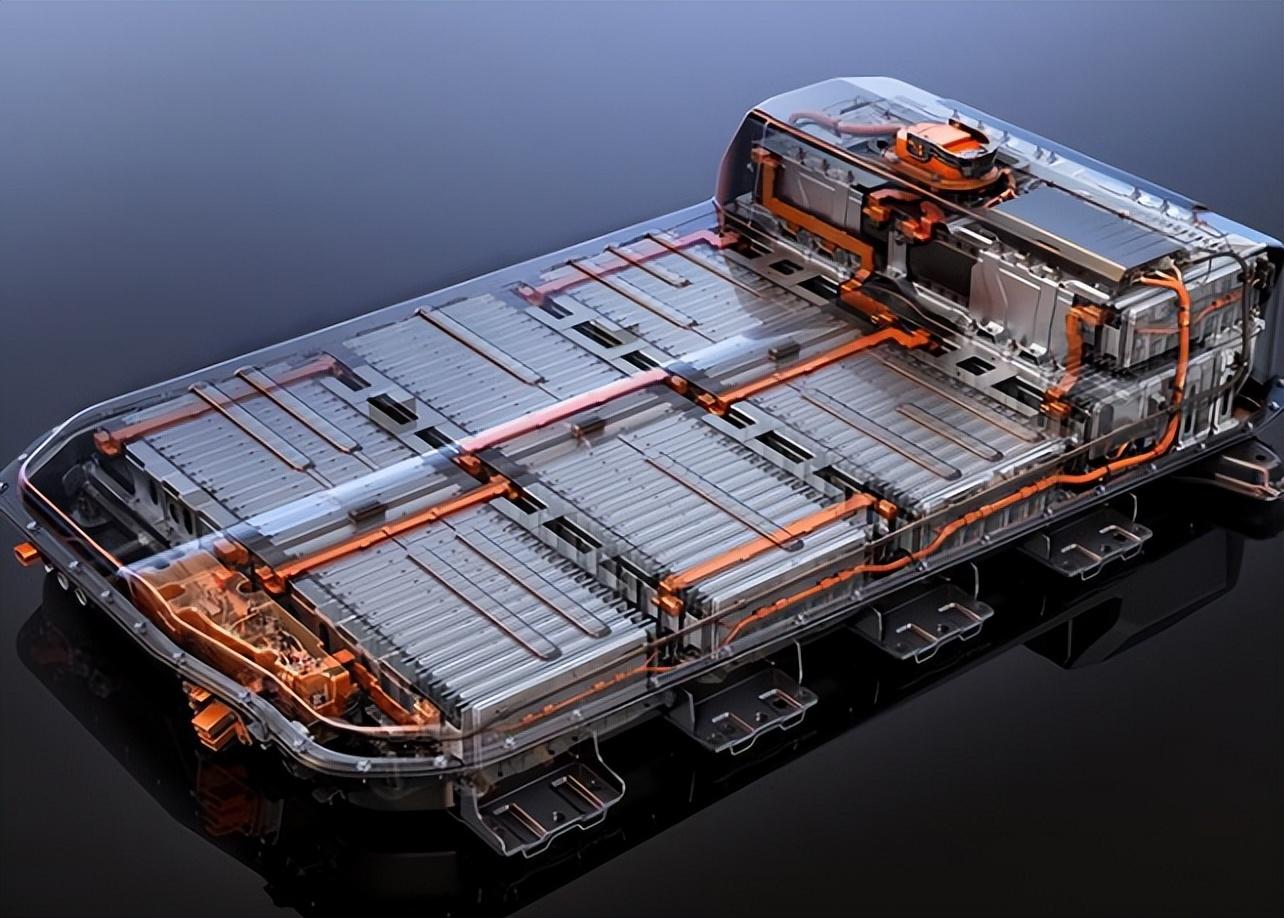
B. Higher Energy Density
Solid-state batteries have the potential to achieve much higher energy densities than traditional lithium-ion batteries. As mentioned earlier, the use of lithium metal anodes in solid-state batteries can significantly increase the energy density of the battery. With a lithium metal anode, the battery can store more lithium ions, which directly translates to a higher capacity and longer-lasting power.
In addition to the lithium metal anode, the solid-state electrolyte can also contribute to higher energy density. Since solid-state electrolytes can be made thinner than liquid-electrolyte-based separators, more active materials (the cathode and anode materials) can be packed into the same volume of the battery. This allows for a greater amount of energy to be stored within the battery. Furthermore, some solid-state electrolytes have better compatibility with high-voltage cathode materials. High-voltage cathodes, such as lithium nickel-manganese-cobalt oxide (NMC) with a high nickel content or lithium cobalt oxide (LCO) at high voltages, can store more energy per unit mass or volume. However, in liquid-electrolyte-based batteries, these high-voltage cathodes can react with the liquid electrolyte, leading to capacity degradation and safety issues. The solid-state electrolyte provides a more stable environment for high-voltage cathodes, enabling their effective utilization and further enhancing the energy density of the battery.
Higher energy density is of great significance for various applications. In electric vehicles, a battery with a higher energy density means a longer driving range, which can address one of the major concerns of consumers, known as “range anxiety.” For portable electronic devices, such as smartphones and laptops, a higher-energy-density battery can provide longer battery life, allowing users to use their devices for extended periods without the need for frequent recharging. In large-scale energy storage systems, higher-energy-density batteries can store more energy in a smaller footprint, reducing the overall cost and space requirements of the storage system.
C. Faster Charging Capability
Solid-state batteries also offer the potential for faster charging compared to traditional lithium-ion batteries. The solid-state electrolyte in these batteries can have relatively high ionic conductivity, especially in the case of certain ceramic electrolytes. High ionic conductivity allows lithium ions to move more quickly between the anode and the cathode during the charging process, enabling a higher current to flow through the battery without significant resistance.
In addition, the use of solid-state electrolytes can reduce the internal resistance of the battery. Internal resistance is a major factor that limits the charging speed of batteries. When a battery is charged, a portion of the electrical energy is dissipated as heat due to the internal resistance. In solid-state batteries, the more efficient ion transport and better contact between the electrodes and the electrolyte can lower the internal resistance, resulting in less heat generation during charging. This not only allows for faster charging but also improves the overall efficiency of the charging process.
Faster charging is a highly desirable feature for many applications. In the context of electric vehicles, long charging times are often a deterrent for consumers. With solid-state batteries, electric vehicles could potentially be charged in a matter of minutes, similar to the time it takes to refuel a gasoline-powered vehicle. This would greatly enhance the convenience and practicality of electric vehicles, accelerating their adoption. For portable electronics, faster charging means that users can quickly top up their device batteries and get back to using them, reducing the downtime associated with charging.
D. Longer Lifespan
Solid-state batteries generally have a longer lifespan compared to traditional lithium-ion batteries. The solid-state electrolyte is more stable over a large number of charge-discharge cycles compared to liquid electrolytes. In liquid-electrolyte-based batteries, the liquid electrolyte can react with the electrodes over time, leading to the formation of a solid-electrolyte interface (SEI) layer on the surface of the electrodes. While the SEI layer initially forms to protect the electrodes from further reaction with the electrolyte, it continues to grow with each charge-discharge cycle, consuming lithium ions and increasing the internal resistance of the battery. This ultimately results in capacity degradation and a shorter lifespan for the battery.
In solid-state batteries, the solid-state electrolyte is less reactive with the electrodes, and the formation and growth of the SEI-like layer are significantly reduced or better controlled. This allows the battery to maintain its capacity and performance over a larger number of charge-discharge cycles. Additionally, the suppression of lithium dendrite growth in solid-state batteries using lithium metal anodes also contributes to a longer lifespan. Dendrites can damage the electrode structure and cause short circuits, leading to premature failure of the battery. By preventing dendrite growth, solid-state batteries can operate reliably for a longer period.
A longer lifespan is beneficial for both consumers and industries. For consumers, it means less frequent battery replacements, which saves money and reduces the environmental impact associated with battery disposal. For industries, such as the electric vehicle industry, longer-lasting batteries can reduce the total cost of ownership for vehicle owners, making electric vehicles more competitive compared to traditional internal combustion engine vehicles. In large-scale energy storage systems, longer-lifespan batteries can increase the return on investment by reducing the frequency of battery replacements and maintenance costs.
E. Wide Operating Temperature Range
Solid-state batteries can operate effectively over a wider temperature range compared to traditional lithium-ion batteries. Liquid electrolytes in lithium-ion batteries have limitations in terms of temperature. At low temperatures, the viscosity of the liquid electrolyte increases, which reduces the mobility of lithium ions, leading to a significant decrease in battery performance. The battery may experience reduced capacity, slower charging speeds, and even failure to start in extreme cold conditions. At high temperatures, the liquid electrolyte can become more volatile and prone to decomposition, increasing the risk of safety issues and accelerating the degradation of the battery.
Solid-state electrolytes, on the other hand, have better thermal stability and can maintain their ionic conductivity over a broader temperature range. Ceramic solid-state electrolytes, for example, can operate well at both low and high temperatures. This wide operating temperature range makes solid-state batteries suitable for a variety of applications in different environmental conditions. In cold regions, electric vehicles equipped with solid-state batteries can maintain better performance, ensuring reliable operation even in sub-zero temperatures. In high-temperature environments, such as in hot deserts or in industrial settings with high-temperature operations, solid-state batteries can avoid the safety and performance issues associated with liquid-electrolyte-based batteries.
F. Improved Form Factor and Design Flexibility
The use of solid-state electrolytes provides greater design flexibility for battery manufacturers. Since solid-state electrolytes are solid and do not require a separate containment structure to prevent leakage, as is the case with liquid electrolytes, batteries can be designed in more complex and unconventional shapes. This allows for better integration of batteries into various devices and systems.
For example, in wearable electronics, where space is extremely limited and the form factor needs to be customized to fit the device's design, solid-state batteries can be manufactured in thin, flexible, or irregular shapes to meet the specific requirements. In electric vehicles, the ability to design batteries with different shapes can optimize the packaging of the battery pack within the vehicle's chassis, potentially improving the vehicle's aerodynamics and interior space utilization. Moreover, the solid-state nature of the battery components also enables the development of thin-film batteries, which have applications in areas such as smart cards, sensors, and other micro-electronic devices where ultra-thin and lightweight power sources are required.
Solid-state batteries represent a significant advancement in battery technology, offering a multitude of advantages over traditional lithium-ion batteries. From enhanced safety and higher energy density to faster charging, longer lifespan, wide operating temperature range, and improved design flexibility, solid-state batteries have the potential to revolutionize various industries, including transportation, consumer electronics, and energy storage. Although there are still some challenges to overcome, such as cost-effective manufacturing and large-scale production, ongoing research and development efforts are rapidly moving solid-state batteries closer to widespread commercialization. As these technologies continue to mature, solid-state batteries are set to play a crucial role in the transition towards a more sustainable and electrified future.
Tel/Whatsapp: 0086-592-7161550

Scan to wechat:
