-
Call Us
0086-592-7161550 -
Email us
ping@aotbattery.com -
Skype
ping@aotbattery.com
Call Us
0086-592-7161550Email us
ping@aotbattery.comSkype
ping@aotbattery.comThe existing secondary batteries have the characteristics of high energy density and good cycling performance. However, the commonly used organic electrolytes are toxic and flammable, which pose certain safety risks. Moreover, the existing electrode materials are limited in resources and have high costs, restricting their large-scale application in the energy storage field. Using water-based electrolytes instead of organic electrolytes can effectively improve the safety performance of the batteries, reduce the production cost, and at the same time, the ionic conductivity of water-based electrolytes is two orders of magnitude higher than that of organic electrolytes, enabling the batteries to have higher power density. Therefore, water-based batteries have received great attention from the industrial and academic communities in recent years.
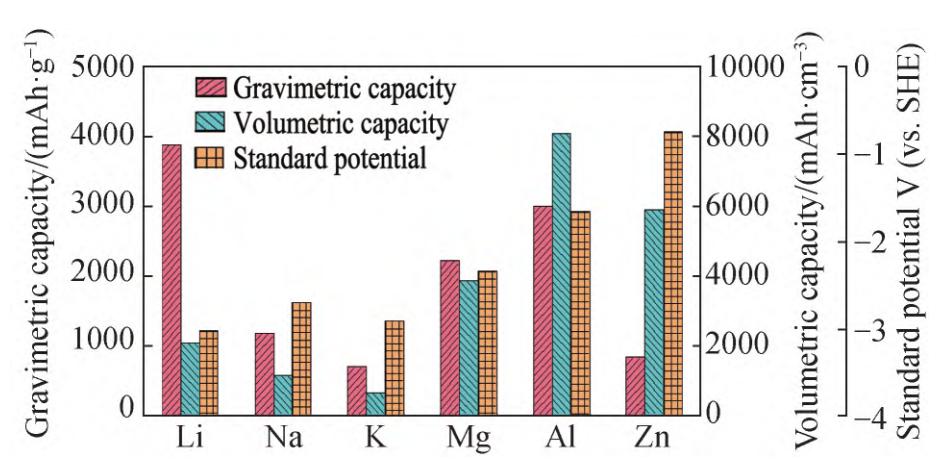
The current water-based batteries mainly include water-based lithium-ion batteries, water-based sodium-ion batteries, and water-based zinc-ion batteries, etc. Comparatively, zinc metal is low-cost, non-toxic, and has a low redox potential, making it more suitable for water-based electrolytes. As the anode of zinc-ion batteries, it has greater research potential. Moreover, due to the high density of zinc and the two-electron reaction involved in the electrochemical reaction, zinc-ion batteries possess higher volumetric energy density and have great application prospects.
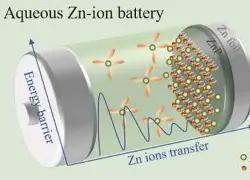
Hydrothermal zinc-ion battery composition
A water-based zinc-ion battery usually consists of water-based electrolyte, separator, and positive and negative electrode materials that provide capacity. The separator is generally made of hydrophilic porous fiber filter paper or glass fiber; the electrolyte is usually a weakly acidic zinc salt solution, such as zinc sulfate (ZnSO4), trifluoromethylsulfonic acid zinc (Zn(CF3SO3)2 or Zn(OTf)2), zinc chloride (ZnCl2), etc., which play the role of transporting charge carriers; the positive electrode material is uniformly mixed with zinc-storing materials, conductive additives and binders, and coated on the current collector to form together. Among them, the zinc-storing materials are some compounds with appropriate redox windows, most of which have layered, framework or tunnel structures; the current collector of the positive electrode generally uses high conductivity materials such as titanium foil, stainless steel mesh, carbon cloth, carbon paper, etc.; the negative electrode usually uses high-purity zinc foil.
Positive electrode material for aqueous zinc-ion batteries
The cathode material serves as the host material for storing Zn2+, and to a large extent, it determines the working voltage and discharge specific capacity of aqueous zinc-ion batteries. Therefore, in order to enhance the competitiveness of aqueous zinc-ion batteries in the large-scale energy storage market, developing cathode materials with excellent electrochemical performance holds significant importance. The charge-discharge performance and cycle stability of the cathode materials have become the most significant factors restricting the large-scale application of batteries at present. Due to the fact that zinc ions easily cause high electrostatic interactions during their insertion and withdrawal in the cathode material, the cathode material needs to balance the characteristics of high capacity and high structural stability. Materials with tunnel structures and large interlayer spacing, such as manganese-based compounds, vanadium-based compounds, and Prussian blue analogues, are currently the most studied cathode materials for aqueous zinc-ion batteries.
Manganese-based compounds possess the characteristics of low toxicity, high voltage, high specific capacity and low cost. In lithium-ion batteries, manganese-based compounds are widely used in positive electrode materials. Due to the limitation of the decomposition voltage of water, the oxidation state of the positive electrode compounds is mainly concentrated on +4 and +3, namely MnO2, Mn2O3 and Mn3O4, as well as the spinel phase ZnMn2O4, MgMn2O4, etc. Among them, MnO2 is the most common and has the highest theoretical specific capacity (308 mAh/g).
Vanadium-based materials are a type of extremely excellent cathode materials for aqueous zinc-ion batteries. The multi-valent vanadium can undergo multiple steps of oxidation and reduction, endowing vanadium-based materials with a relatively high theoretical specific capacity. Moreover, vanadium-based materials exhibit appropriate voltage platforms, approximately 0.8 - 1.0 V (vs. Zn/Zn2+). At the same time, the structure formed by the interconnection of easily deformable V-O polyhedra gives vanadium-based materials strong designability. Currently, various types of vanadium-based materials have been used as cathode materials for aqueous zinc-ion batteries, including: vanadium oxides (V2O5, VO2, V2O3, V6O13, etc.), vanadium salts (NaV3O8·1.5H2O, LiV3O8, CuV2O6, etc.), vanadium-based materials with poly-anionic framework (NASICON) structure, and other vanadium-based sulfides and vanadium-nitrogen oxides. They have higher capacity than manganese-based compounds, but have lower discharge voltages.
Prussian blue analogues are host materials for various metal ions. Cubic metal-organic compounds have the characteristics of wide intercalation channels and high ionic conductivity. In prussian blue analogues, the metal hexacyanoferrate (HCF) is the most promising candidate material, which is a suitable host material for monovalent and polyvalent ions. For aqueous zinc-ion batteries, the reversible intercalation processes of ZnHCF and CuHCF in ZnSO4 electrolyte have been proven to be reversible. Moreover, prussian blue analogues have a very high positive electrode potential. At 1.7V vs. Zn/Zn2+, the potential is close to the oxygen reduction reaction potential in aqueous electrolytes. Although they make good use of the stability window of the electrolyte, the specific capacity of all prussian blue analogues is relatively low, approximately 60mAh/g, which is inferior to manganese-based and vanadium-based compounds.
In addition to the above several types of materials, other organic materials, transition metal sulfides and oxides with appropriate voltage windows and suitable structures have also become hotspots in zinc storage research, such as: organic materials like polyaniline and quinone compounds; Mo6S8, MoS2 and other molybdenum-based materials; Co3O4 and other cobalt-based materials; metal-organic framework materials (MOFs), covalent organic frameworks (COFs); Mxene layered materials, etc.
Problems and Optimization of Cathode Materials for Water-Based Zinc-Ion Batteries
Manganese-based cathode materials generally suffer from poor electronic conductivity, slow Zn2+ reaction kinetics, volume expansion during cycling, and cathode material dissolution. These issues lead to rapid capacity decay and poor cycling stability, thereby reducing their application value in battery systems. To address these problems, methods such as defect engineering, interface modification, cation doping, and composite material construction are commonly employed for improvement.
Vanadium-based cathode materials currently face issues such as high diffusion resistance of Zn2+ in the electrode, solubility of vanadium in aqueous electrolytes, and unstable structure. Researchers have conducted extensive studies by means of intercalation treatment, structural and morphological optimization, composite with high conductivity substances, defect introduction, or electrolyte optimization to enhance battery capacity and cycling stability. At the same time, efforts are being made to research new vanadium-based cathodes and explore the application of vanadium oxides, vanadates, and phosphovanadium compounds as cathode materials for aqueous zinc-ion batteries.
Prussian blue analogues suffer from poor conductivity and few active sites, resulting in low capacity and reduced energy density. Additionally, the phase transformation process of the electrode during cycling also leads to capacity decay and a decrease in cycling performance. To solve these problems, vacancies are typically introduced into the crystal framework or other redox active sites to provide additional capacity.
Tags :
Tel/Whatsapp: 0086-592-7161550

Scan to wechat:
