-
Call Us
0086-592-7161550 -
Email us
ping@aotbattery.com -
Skype
ping@aotbattery.com
Call Us
0086-592-7161550Email us
ping@aotbattery.comSkype
ping@aotbattery.com
SEI (Solid Electrolyte Interphase) is also a solid electrolyte interface membrane.
When a lithium-ion battery is charged and discharged for the first time, a small amount of polar aprotic solvent in the electrolyte undergoes a reduction reaction after gaining some electrons, and combines with lithium ions to form an interface film with a thickness of about 100-120nm, which is SEI. SEI is usually formed at the solid-liquid interface between the lithium battery electrode material and the lithium battery electrolyte.
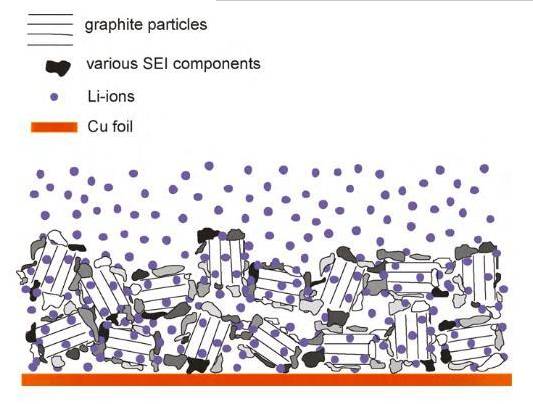
When the lithium-ion battery starts to charge and discharge, the lithium ions are extracted from the positive electrode active material, enter the electrolyte, penetrate the lithium battery separator and then enter the electrolyte, and finally insert into the layered voids of the negative electrode carbon material, and the lithium ions complete a complete de-intercalation behavior.
At this time, electrons come out from the positive electrode along the outer loop and enter the negative electrode carbon material. A redox reaction occurs between the electrons, the solvent in the electrolyte and the lithium ions.
After receiving the electrons, the solvent molecules combine with the lithium ions to form SEI and generate H2, CO, CH2=CH2 and other gases. As the thickness of the SEI increases until the electrons cannot penetrate, a passivation layer is formed, which inhibits the redox reaction from continuing.
Tel/Whatsapp: 0086-592-7161550

Scan to wechat:
